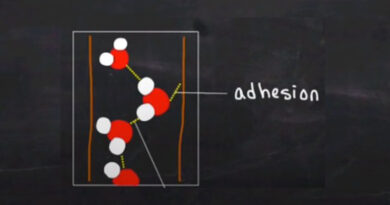
What is Matter? Definition of matter
What is matter? Matter is defined as any substance that has mass and occupies space by having volume in classical physics and general chemistry. All everyday items that can be hit are finally made up of atoms, which are made up of engaging subatomic particles. The “matter” refers to atoms and anything made up of them, as well as any particles that behave as if they have respectively rest mass and volume, in both every day and scientific usage.
However, it excludes mass-less particles like photons, as well as other energy phenomena or waves like light and heat. There are many different states/phases of matter (What is matter?). Other states, such as plasma, Bose–Einstein condensates, fermionic condensates, and quark–gluon plasma, are possible in addition to the classic everyday phases of solid, liquid, and gas. For example, water is found as ice, liquid water, and gases steam – but other states, such as plasma, Bose–Einstein condensates, fermionic condensates, and quark–gluon plasma, are also possible.
Atoms are typically thought of as having a nucleus of protons and neutrons, as well as a “cloud” of orbiting electrons that “take up space.” However, this is only partially right because subatomic particles and their properties are determined by their quantum nature, which means they do not behave in the same way that ordinary objects do. They can behave like waves as well as particles and do not have clearly-defined sizes or positions.
Matter is not a basic concept in the Standard Model of particle physics since the fundamental ingredients of atoms are quantum objects with no intrinsic “size” or “volume” in any sense of the word. Under everyday conditions, some “point particles” known as fermions (quarks, leptons), as well as many components and atoms, are successfully obliged to continue a distance from other particles due to the isolation principle and other fundamental relations; this produces the property of matter, which looks to us as matter taking up space.
People have pondered the exact nature of matter for most of the history of natural science. In the first millennium BC, Buddhists, Hindus, and Jains in ancient Greece and India independently developed the idea that matter was made up of distinct building units, known as the particulate theory of matter. Kanada is one of the ancient thinkers who suggested the particle theory of matter.