What is activation energy? Catalyst in Activation Energy
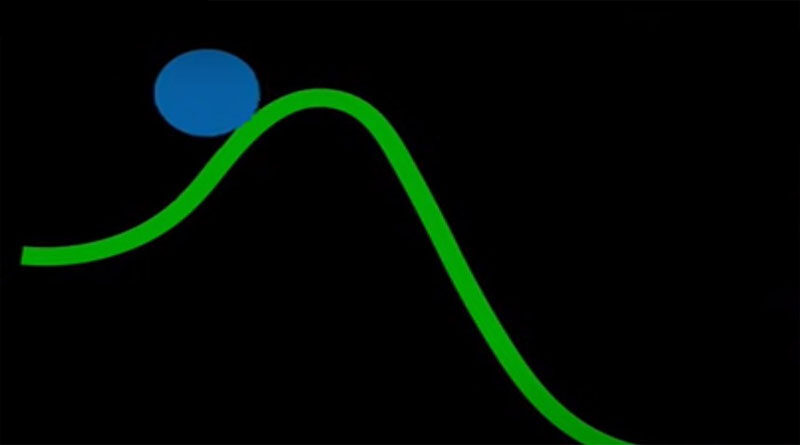
In chemistry and physics, activation energy refers to the lowest amount of energy required for a chemical reaction to occur. It is also called bond energy. The activation energy of a reaction calculates in joules per mole (J/mol), kilojoules per mole (kJ/mol), or kilocalories per mole (kcal/mol). The magnitude of the potential barrier (also known as the energy barrier) divides the minima of the potential energy surface corresponding to the original. Activation energy calls the final thermodynamic states.
The heat should be high enough for a significant number of molecules with transverse energy equal to or higher than the binding energy to proceed at a tolerable rate in a chemical reaction. Svante Arrhenius, a Swedish scientist, proposed the word “activation energy” in 1889.
Bond energy is also applicable to nuclear processes and a variety of other physical processes.
Functions of Catalyst in Activation Energy
A catalyst is a material that affects the thermodynamic equilibrium to lower the activation energy. An enzyme act as a catalyst that makes entirely of protein and small-molecule co-factors. The catalyst increases the rate of a reaction. In the reaction, the catalyst remains stable.
Furthermore, while the catalyst reduces the binding energy, it has no effect on the energies of the original reaction mixture, and so has no effect on the equilibrium. Rather, the change is only the binding energy, while the reactant and product energies hold stable (lowered).
The production of a more favorable transition state reduce the activation energy of a catalyst. Catalysts, by their very nature, make it easier for a reaction’s substrate to advance to a transition state. This makes possible by the release of energy that happens when a substrate attaches to a catalyst’s active site.
In some circumstances, when the temperature rises, the rate of reaction decreases. This results in a negative number when following an essentially exponential distribution. The rate constant can even be fit to an Arrhenius equation of binding energy.